James Chadwick was an English Physicist who was awarded the 1935 No...
The identification of the neutron was to have important ramificatio...
In 1930, Bothe and Becker observed that the bombardment of berylliu...
The Cavendish Laboratory at Cambridge University was the home of so...
Chadwick was able to prove that the neutral particle could not be a...
The task which remained for Chadwick was that of determining the ma...
Chadwick was able to prove that the neutral particle could not be a photon by bombarding targets other than hydrogen, including nitrogen, oxygen, helium and argon. Not only were these inconsistent with photon emission on energy grounds, the cross-section for the interactions was orders of magnitude greater than that for Compton scattering by photons.
In 1930, Bothe and Becker observed that the bombardment of beryllium with alpha particles from a radioactive source produced neutral radiation which was penetrating but non-ionizing. They presumed it was gamma rays, but Curie and Joliot showed that when you bombarded a paraffin target with this radiation, it ejected protons with energy about 5.3 MeV.

This proved to be inconsistent with gamma rays, as can be shown from momentum and energy analysis:
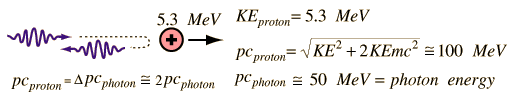
This analysis follows that for a headon elastic collision where a small particle strikes a much more massive one. Again, the necessary energy for the gamma ray explanation was much greater than any energy observed to be available from the nucleus (gamma rays are produced during the decay of an atomic nucleus from a high energy state to a lower energy state and they are almost always less than 10MeV), so the neutral radiation must be some kind of neutral particle.
The Cavendish Laboratory at Cambridge University was the home of some of the great discoveries in physics. It was founded in 1874 by the Duke of Devonshire (Cavendish was his family name), and its first professor was James Clerk Maxwell.

The talent density was so high that this photograph of the Staff and Research Students of the Cavendish Laboratory in 1933 (around the time this paper was published) included six Nobel Laureates: [Chadwick](https://en.wikipedia.org/wiki/James_Chadwick), [JJ Thomson](https://en.wikipedia.org/wiki/J._J._Thomson), [C.T.R Wilson](https://en.wikipedia.org/wiki/Charles_Thomson_Rees_Wilson), [Rutherford](https://en.wikipedia.org/wiki/Ernest_Rutherford), [Patrick Blackett](https://en.wikipedia.org/wiki/Patrick_Blackett) and [Ernest Walton](https://en.wikipedia.org/wiki/Ernest_Walton). 12% of the people in this photo were Nobel Laureates!

James Chadwick was an English Physicist who was awarded the 1935 Nobel Prize in Physics for his discovery of the neutron in 1932. He was also the head of the British team that worked on the Manhattan Project during World War II.

Chadwick was born near Manchester of a poor family. He was a hard worker at school and
obtained a scholarship to attend university. Preliminary interviews were held at Manchester University
with physics and mathematics entrants being interviewed in the same building but he got in the wrong
line and found himself answering questions from a physics department lecturer. As he was too shy to
admit that he wanted to study mathematics but because he also liked the enthusiasm of his interviewer, he
ended up going to university in 1908 to study physics.
His qualities were recognised by [Rutherford](https://en.wikipedia.org/wiki/Ernest_Rutherford), the father of nuclear physics, who was Chadwick's advisor and who later invited him to work at the Cavendish Laboratory where the discovery of the Neutron was made.
The identification of the neutron was to have important ramifications in numerous fields. Two years after Chadwick's discovery, [Walter Baade](https://en.wikipedia.org/wiki/Walter_Baade) and [Fritz Zwicky](https://en.wikipedia.org/wiki/Fritz_Zwicky) proposed the formation of [neutron stars in supernovae](https://en.wikipedia.org/wiki/Neutron_star). They had been the first to describe a supernova the previous year.
In 1933, [Leo Szilard](https://en.wikipedia.org/wiki/Leo_Szilard) realised that a neutron would more easily interact with a nucleus than would an alpha particle and if a reaction involving a neutron with a nucleus could result in two or more neutrons being produced, this might make a chain reaction possible. This finding was fundamental in the development of the atomic bomb.

Fig: Albert Einstein and Leo Szilard
The task which remained for Chadwick was that of determining the mass of the neutral particle. Through conservation of momentum techniques, he was able to determine that the mass of the neutral radiation was almost exactly the same as that of a proton. This is Chadwick's equation:
$$
^{4}_{2}\alpha + ^{9}_{4}Be \rightarrow ^{12}_{6}C+^{1}_{0}n
$$
Since energy must be conserved:
$$
\frac{1}{2}m_{\alpha}v^2_{\alpha}+m_{\alpha}c^2+m_{Be}c^2 = \frac{1}{2}m_{C}v^2_{C} + m_{C}c^2+\frac{1}{2}m_{n}v^2_{n} + m_{n}c^2
$$
Solving for the velocity of the neutron gives
$$
v_n = \sqrt{\frac{\frac{1}{2}m_{\alpha}v^2_{\alpha}+m_{\alpha}c^2+m_{Be}c^2-m_{C}c^2 - \frac{1}{2}m_{C}v^2_{C}-m_{n}c^2}{\frac{m_n}{2}}}
$$
Assuming that the neutron mass was close to that of the proton, Chadwick was able to arrive at values that are consistent with the experiment - $3.2 \times 10^9 cm/s$!